STUDY PROTOCOLS
Smoking cessation intervention prior to orthopedic surgery: A study protocol to determine patient outcomes and feasibility
More details
Hide details
1
Department of Acute Medicine,
Päijät-Häme Central Hospital,
Lahti, Finland
2
Institute of Public Health and
Clinical Nutrition, University of
Eastern Finland, Kuopio, Finland
3
Department of Public Health
and Welfare, National Institute
for Health and Welfare, Helsinki,
Finland
4
Research Unit, Orton
Orthopedic Hospital, Helsinki,
Finland
5
Surgical Outpatient
Department, Päijät-Häme Central
Hospital, Lahti, Finland
6
Department of Health Security,
National Institute for Health and
Welfare, Helsinki, Finland
7
Faculty of Medicine,
Department of Nursing Science,
University of Turku, Turku,
Finland
8
Institute for Molecular
Medicine Finland FIMM,
University of Helsinki, Helsinki,
Finland
9
Department of Administration,
Tampere University Hospital,
Tampere, Finland
Submission date: 2022-01-14
Final revision date: 2022-02-15
Acceptance date: 2022-08-03
Publication date: 2022-08-30
Corresponding author
Antti Kyrö
Surgical Outpatient
Department, Päijät-Häme Central
Hospital, Keskussairaalankatu 7,
15850 Lahti, Finland
Tob. Prev. Cessation 2022;8(August):33
KEYWORDS
TOPICS
ABSTRACT
The aim of the study is to analyze the effect of smoking and smoking cessation on
the incidence of complications among orthopedic and hand surgery patients, and
to determine the feasibility of smoking cessation intervention, as well as factors
predicting success in smoking cessation. Orthopedic and hand surgery patients will
be invited to participate in the study, which will recruit 550 participants (at least
20% daily smokers). A participant will be defined as a daily smoker if he/she reports
daily smoking and/or laboratory tests show active smoking. Data will be collected
using a self-reported questionnaire and from medical records. Smokers will receive
information about the benefits of smoking cessation and will be encouraged to
quit. Medication or nicotine replacement therapy will be prescribed. Laboratory
tests will be taken two weeks before and two weeks after surgery. Follow-up phone
calls will be made at 3, 6, and 12 months after surgery. The primary outcome is
any complication, defined as a prolonged stay in hospital or any additional visit
to or measure taken by a health service during the 12 months after surgery. Data
on complications are mainly obtained from personal health records and from the
information received at the follow-up; the rest of the data will be collected from the
register of healthcare-associated infections. Secondary outcomes are the number
and types of complications. The sample (n=550) was calculated to observe a 10%
difference in complications between smokers and non-smokers (5% alpha level and
80% power), considering a 10% drop-out rate. Logistic regression and log-linear
models will be used for data analyses.
INTRODUCTION
Smoking has many adverse effects on orthopedic and hand surgery patients. It impairs blood circulation in the area of surgical intervention1,2, oxygenation of tissues3,4, and immune response to infections5,6. Smoking increases the risk of thrombosis in vessels7,8. Further, it impairs the function of osteoblasts9, decreases the resorption of calcium from the intestines10, and lowers the level of vitamin D5.
Smoking also hinders wound healing11. Tibial, femoral, humeral, clavicular, and wrist navicular fractures heal more slowly in smokers than in non-smokers12-17. The healing of ankle arthrodesis18, high tibial osteotomy19, and ulnar shortening osteotomy20, is also slower and more uncertain among smokers than among non-smokers. Smokers have more complications on hip and knee arthroplasties compared with non-smokers21,22. The outcome of spinal surgery is worse for smokers than for non-smokers23,24. Smokers have six-fold overall risk of surgical wound infections compared with that of non-smokers25.
Nåsell et al.26 have shown that in the smoking cessation intervention group, 20% compared with 38% in the control group had surgical complications after acute fracture surgery. In another study by Møller et al.27, the overall complication rate was 18% in the smoking cessation intervention group and 52% in the control group, and the median length of hospital stay was 11 and 13 days, respectively. Glassman et al.28 have shown that smoking cessation for longer than six months after a posterior instrumented lumbar spinal fusion reduces the rate of nonunion to 17% compared with 26% for those who continued to smoke after surgery. The nonunion rate was 14% among non-smokers. In a study of elective orthopedic and special surgery patients with a smoking cessation intervention of four weeks before and four weeks after surgery, the incidence of postoperative complications was 21% in the intervention group and 41% in the control group29.
Hejblum et al.30 have shown in their cost-benefit analysis that a smoking cessation intervention of hip and knee arthroplasty patients saved on average 117 € per patient in 2008. The average 90-day cost of total joint arthroplasty with a smoking cessation intervention was 32 US$ less than the respective cost without intervention in 201731. In the US, the mean cost of a total hip arthroplasty with a periprosthetic infection was over 88 thousand US$ and that without a periprosthetic infection over 25 thousand US$, from 2007 to 201132. So, the mean cost of a periprosthetic infection after a total hip arthroplasty was close to 63 thousand US$ . In all, smoking cessation preoperatively is a very valuable and cost-effective method of reducing the risks of postoperative complications, especially infections of orthopedic patients. With this intervention, a significant amount of money could be saved. Further, smoking cessation is a very effective method to improve health33. Tosi et al.34 have reported that an intervention program among patients who had a low-energy fracture and who received counselling on calcium and vitamin-D supplementation, weight-bearing exercise, smoking cessation, and fall prevention, reduced the risk of secondary fractures.
It is very challenging to motivate smokers to quit before orthopedic or hand surgery. This is even though we know very well that the risk of complications is much lower when a patient stops smoking before surgery. Lindström et al.29 found that a significant proportion of their patients were not interested in smoking cessation and they wanted only to concentrate on the forthcoming surgery. Bender et al.35 showed, in their study of patients who had surgery for long bone nonunion, that only 8% of smokers had stopped smoking at six weeks after surgery.
Aims
This article describes the protocol of our study which aims to determine: 1) the effects of smoking and smoking cessation intervention on the incidence of complications after orthopedic surgery; 2) the feasibility of preoperative smoking cessation intervention and its effect on reducing smoking; and 3) which factors predict success in smoking cessation. The secondary aims of the study are: a) to compare the incidence of different types of complications in the whole study population and in different subgroups of orthopedic and hand surgery patients who are smokers, non-smokers or quitters who stopped smoking during the preoperative intervention; and b) to determine which other factors in addition to smoking, such as chronic diseases and medication, explain the incidence of complications.
Hypotheses
Smoking patients have more complications compared with non-smoking patients and patients continuing smoking despite smoking cessation intervention have more complications than initially smoking patients who are stopping smoking because of the intervention.
Smoking cessation of patients with planned arthroplasties or heavy spine operations or other heavy operations, and of patients with low or moderate nicotine dependence, is successful more often than in other patients.
METHODS
Recruitment
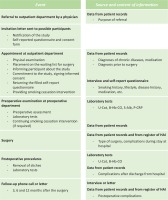
Participants for the study will be recruited among patients visiting the orthopedic or hand surgery outpatient department and who are on the waiting list for elective orthopedic or hand surgery in Päijät-Häme Central Hospital, Lahti, Finland (Figure 1). The orthopedic surgeon (who registers the patient on a waiting list) and nurse decide if the patient is eligible to participate in the study.
Figure 1
The eligibility criteria are at least 18 years of age and that the planned surgery will be directed at the bone. Patients under 18 years of age, patients with an acute fracture, and patients awaiting other types of surgery are excluded from the study. A total of 550 patients will be recruited aiming to have at least 20% of them daily smokers, corresponding approximately to the current smoking prevalence in Finland36. The number of smokers will be followed up during the process so that more could be recruited during the recruitment period if their proportion seems too small.
Data collection
Participants fill in a self-reported questionnaire collecting detailed background information on chronic diseases, medication, socio-economic status, physical activity, and use of alcohol. The questionnaire includes questions on present and past smoking; use of electronic cigarettes, snus, and pipe tobacco; and exposure to passive smoking. Additional and clarifying information such as diagnoses of chronic diseases and prescribed medication will be collected from participants’ personal health records. Information on the orthopedic diagnosis and planned orthopedic surgery will be filled in on the study form (Figure 1).
During the preoperative assessment at about two weeks before the surgery, laboratory tests will be taken to verify the smoking status of the participants and to differentiate the use of other nicotine products (by cotinine of urine, U-Cot and carboxyhaemoglobin, Hb-CO) and to exclude malnutrition (albumin of serum, S-Alb) and other significant catabolic processes (C-reactive protein, P-CRP) (Figure 1). Participants’ smoking history will be based on classification used in surveys conducted by the Finnish National Institute for Health and Welfare (THL) and categorized from 1 to 5 as follows: 1=daily smoker, 2=occasional smoker, 3=quit smoking 1–12 months ago, 4=quit smoking over 12 months ago, and 5=never smoker36.
During the intervention, participants’ smoking status will be confirmed using the following criteria. A participant is determined as a smoker if he/she reports daily smoking and/or U-Cot is above 250 µg/L and Hb-CO above 2%. A participant who reports exposure to passive smoking and/or has a U-Cot of 100–250 µg/L, is categorized as a passive smoker. Among non-smokers, U-Cot is typically <50 µg/L and Hb-CO <2%. Hb-CO is used to specify users of snus or NRT, among whom Hb-CO is <2% but U-Cot is elevated.
Intervention
Smokers will be given verbal and written information about health and economic benefits of smoking cessation and encouraged to quit smoking. The Fagerström test for nicotine dependence (FTND) will be conducted to estimate the level of nicotine dependence of smokers37. If a smoker has moderate nicotine addiction, NRT will be recommended. Prescription medication to aid smoking cessation will be considered if a participant had strong nicotine dependence, i.e. smoked over 10 cigarettes per day and first cigarette was lit within 30 minutes after waking up, or a previous attempt to quit smoking yielded significant withdrawal symptoms38.
The smoking cessation intervention will be given in the outpatient department, by the surgeon, the nurses, or both. In discussions with smokers, the aim is to share information, persuade, motivate, and offer skills training and social support to quit smoking. The intervention will be performed during an outpatient appointment when the patient is registered on the waiting list and also during the preoperative visit. After surgery, when the stitches will be removed, Hb-CO and U-Cot tests will be repeated to verify participants’ smoking status to verify successful smoking cessation. Participants will be followed up to 12 months after surgery. Participants will receive a phone call or letter at 3, 6, and 12 months after surgery, asking them if any problems or complications had occurred (Figure 1). The description and ICD codes of complications will be recorded in the study form.
Data from operation and definition of complications
The Finnish register of healthcare-associated infections (HAI) includes information about the operations of all surgically treated patients, regardless of whether the patient has had a postoperative infection or not. For this study, information about the surgery and any postoperative infection will be obtained from that register. A complication will be defined as any event which causes additional medical or surgical treatment, an additional radiological or laboratory test, a prolonged stay in hospital or any additional visit to a healthcare unit, following the criteria used by Nåsell et al.26. Data on complications have been mainly collected and will be completed from participants’ personal health records during their perioperative stay in hospital. These data will be combined with all available personal health data during 12 months after surgery and the information received during follow-up phone calls at 3, 6, and 12 months after surgery (Figure 1).
Primary and secondary outcomes
The primary outcome is any complication occurring within 12 months after surgery. The number and types of complications (for example rapidly occurring complications, repeat surgery, and infections, stratified by types of surgery) are considered as secondary outcomes. Subgroup analysis based on patient characteristics and comorbidities will be carried out. Successful smoking cessation is a feasibility outcome.
Sample size calculation and power analysis
The sample size was calculated assuming that the proportion of smokers in the patient population is comparable with the smoking prevalence in the Finnish adult population, i.e. about 20%36. The other assumption was that with appropriate support before surgery, as many as half of the smokers could quit smoking. The sample (n=550) was calculated to observe 10% difference in postoperative complications between smokers and non-smokers, with 5% alpha error and 80% power, considering a 10% drop-out rate.
Data analyses
To analyze the risk of primary outcome, i.e. the postoperative complications among smokers, non-smokers, and quitters, a logistic regression model will be used. The effectiveness of the smoking cessation intervention will be assessed using logistic regression analyses. Associations of smoking status and background variables with complications will be analyzed using log-linear models considering patients’ gender, age, and comorbidities.
Study ethics procedures
Participation in the study is voluntary. All eligible patients will be informed about the study and motivated to participate by an invitation letter. Those willing to participate will be given a detailed information brochure and asked to sign an informed consent form. Ethical approval for the study was received from the Regional Ethical Committee of Tampere University Hospital, Finland (Approval number: R15129; Date: 22 September 2015). Permission for the study was granted by the chief medical director of Päijät-Häme Central Hospital, Lahti, Finland.
Patient and public involvement statement
Neither patients nor members of the public were and will be involved in the design or conduct of this study.
DISCUSSION
In studies concerning smoking and orthopedic surgery, the definition of smoker and/or smoking status has been diverse. For example, ex-smokers are classified as non-smokers; mostly, smoking status is based only on patients’ self-report without any biochemical verification. Bender et al.35 showed in their study of patients who had had surgery for long bone non-union that 23% of participants reporting to be former smokers and 6% of participants reporting to be non-smokers had serum cotinine values suggesting that they were active smokers. In this study, laboratory tests will be performed to biochemically verify participants’ smoking status to increase the evidence for the effect of smoking on the outcome of orthopedic surgery. Thus, some participants who report to be non-smokers could be classified as active smokers based on the laboratory tests.
Ehnert et al.39 showed that the rate of complications after joint arthroplasty of smokers exceeds that of non-smokers and increasing number of cigarettes smoked raises the rate of complications. Also, they showed that bone formation and inflammatory response markers of smokers are lower than those of non-smokers. However, the aim of this study is to gather evidence on the effects of biochemically confirmed smoking and smoking cessation on the outcome of defined, but otherwise unselected orthopedic and hand surgery operations and on feasibility and effects of a preoperative smoking cessation intervention. The intervention will be carried out in real-life settings, so all smokers will receive the smoking cessation intervention following a standard protocol. Randomization to smoking cessation intervention (yes or no) among active smokers would be an unethical study design. Further aims are to assess the factors affecting the feasibility and outcome of the intervention and its effects on costly complications which are more likely to occur among smokers than non-smokers. This valuable information could be used in future to support orthopedic or hand surgery patients in smoking cessation.
Strengths and limitations
The strengths include that the study population will be recruited from a central hospital serving the whole population of a catchment area. Further, laboratory tests will be taken to biochemically verify participants’ smoking status to increase accuracy of smoking status assessment among the patients. A potential limitation is that the smoking cessation interventions will be carried out during the clinical work in the orthopedic and hand surgery outpatient departments and surgical wards at the non-teaching central hospital. Further, because of previous evidence of effectiveness of smoking cessation intervention, randomized trial design was not feasible. Finally, there will be probably overrepresentation of non-smoking patients for the study due to the requirement of filling a long questionnaire, complying with extra laboratory tests to be taken twice, and answering follow-up phone calls three times presumed during the study.
CONCLUSIONS
This study will provide new and valuable information on smoking cessation in reducing complications in surgery and will also demonstrate feasibility of smoking cessation intervention in a hospital treating orthopedic and hand surgery patients.
ACKNOWLEDGEMENTS
The authors thank A. L. Kankainen for conducting the sample size
calculation and power analysis and for valuable help during the study; V.
Lehtinen for his promise to provide in the future participant data from the
register of HAI; T. Vasankari for co-operation and help during the study;
senior orthopedic surgeon J. Haapala for giving permission for the study
and for making it possible; all orthopedic and hand surgeons and assistant
surgeons for recruiting participants and for co-operation; P. Puska for
help and advice during the study; A. Braun for performing the follow-up;
K. Granlund for entering data into the database; all nurses at the surgical
outpatient department for recruiting participants and the head nurses for
their co-operation; all nurses at the preoperative department and nurses at
the orthopedic department for their co-operation; and K. Sotejeff-Wilson
for revision of the English language of an earlier version of the manuscript.
CONFLICTS OF INTEREST
The authors have each completed and submitted an ICMJE Form for
Disclosure of Potential Conflicts of Interest. The authors declare that they
have no competing interests, financial or otherwise, related to the current
work. A. Kyrö reports grants from Bioretec Ltd and Erkki Poikosen säätiö,
Erkki Poikonen’s Foundation, outside the submitted work.
FUNDING
This work is supported by research funding of the government of Finland
(State Research Funding) grant numbers D/1082/02.05.01.00.03/2017 and
D938/02.05.01.00.03/2018. The study is also supported by the Foundation
of the Finnish Anti-Tuberculosis Association, Doctors Against Tobacco
Network, Finnish Lung Health Association (FILHA Ry), Erkki Poikonen’s
Foundation (Erkki Poikosen säätiö), and Päijät-Häme Doctors’ Association.
The funders had no role in the study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
ETHICAL APPROVAL AND INFORMED CONSENT
Ethical approval for the study was received from the Regional Ethical
Committee of Tampere University Hospital, Finland (Approval number:
R15129; Date: 22 September 2015). Permission for the study was granted
by the chief medical director of Päijät-Häme Central Hospital, Lahti,
Finland. Those willing to participate will be given a detailed information
brochure and asked to sign an informed consent form.
DATA AVAILABILITY
The data supporting this research cannot be made available for privacy
reasons.
AUTHORS' CONTRIBUTIONS
AK conceptualized the study. KK, AK, AM, SST, MH, TK and ML designed the
study. KK, AK, SST, ML and MH drew up the study and questionnaire forms.
SST, AK, MH and ML recruited participants. SST, AK, ML and MH carried
out the smoking cessation interventions. SST, ML, MH and AK did the
follow-up. KK and ML collected personal health data from participants and
entered them in the database. KK, AK, AM, TL and ML interpreted the data.
KK drafted the manuscript; AK, TL, AM, TK, JS and ML revised it; all authors
read and approved the final manuscript.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
PROTOCOL REGISTRATION
The protocol is registered on ISRCTN registry with study ID
ISRCTN16961081.
REFERENCES (39)
1.
Uehara K, Sone R, Yamazaki F. Cigarette smoking following a prolonged mental task exaggerates vasoconstriction in glabrous skin in habitual smokers. JUOEH. 2010;32(4):303-316. doi:10.7888/juoeh.32.303
2.
Avery MR, Voegeli D, Byrne CD, Simpson DM, Clough GF. Age and cigarette smoking are independently associated with the cutaneous vascular response to local warming. Microcirculation. 2009;16(8):725-734. doi:10.3109/10739680903199194
3.
Kimura Y, Nakamoto Y, Shitama H, Ohmine S, Ide M, Hachisuka K. Influence of moderate smoking on physical fitness and local muscle oxygenation profile during incremental exercise. JUOEH. 2007;29(2):149-158. doi:10.7888/juoeh.29.149
4.
Kastelein TE, Duffield R, Crowcroft S, Marino FE. Cerebral oxygenation and sympathetic responses to smoking in young and middle-aged smokers. Hum Exp Toxicol. 2017;36(2):184-194. doi:10.1177/0960327116641736
5.
Abate M, Vanni D, Pantalone A, Salini V. Cigarette smoking and musculoskeletal disorders. Muscles Ligaments Tendons J. 2013;3(2):63-69. doi:10.11138/mltj/2013.3.2.063
6.
Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83(1-2):148-156. doi:10.1016/s0165-5728(97)00231-2
7.
Roy S. Effects of smoking on prostacyclin formation and platelet aggregation in users of oral contraceptives. Am J Obstet Gynecol. 1999;180(6Pt2):S364-S368. doi:10.1016/s0002-9378(99)70697-6
8.
Lutsey PL, Virnig BA, Durham SB, et al. Correlates and consequences of venous thromboembolism: The Iowa Women's Health Study. Am J Public Health. 2010;100(8):1506-1513. doi:10.2105/AJPH.2008.157776
9.
Holzer N, Braun KF, Ehnert S, et al. Green tea protects human osteoblasts from cigarette smoke-induced injury: possible clinical implication. Langenbecks Arch Surg. 2012;397(3):467-474. doi:10.1007/s00423-011-0882-8
10.
Need AG, Kemp A Giles N, Morris HA, Horowitz M, Nordin BE. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13(1):83-88. doi:10.1007/s198-002-8342-9
11.
Theocharidis V, Katsaros I, Sgouromallis E, et al. Current evidence on the role of smoking in plastic surgery elective procedures: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2018;71(5):624-366. doi:10.1016/j.bjps.2018.01.011
12.
Moghaddam A, Zimmermann G, Hammer K, Bruckner T, Grützner PA, von Recum J. Cigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fractures. Injury. 2011;42(12):1435-1442. doi:10.1016/j.injury.2011.05.11
13.
Kyrö A, Usenius J-P, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262. PMID:8122874.
14.
Spross C, Platz A, Rufibach K, Lattmann T, Forberger J, Dietrich M. The PHILOS plate for proximal humeral fractures--risk factors for complications at one year. J Trauma Acute Care Surg. 2012;72(3):783-792. doi:10.1097/TA.0b013e31822c1b5b
15.
Murray IR, Foster CJ, Eros A, Robinson CM. Risk factors for nonunion after nonoperative treatment of displaced midshaft fractures of the clavicle. J Bone Joint Surg Am. 2013;95(13):1153-1158. doi:10.2106/JBJS.K.01275
16.
Ricci WM, Streubel PN, Morshed S, Collinge CA, Nork SE, Gardner MJ. Risk factors for failure of locked plate fixation of distal femur fractures: an analysis of 335 cases. J Orthop Trauma. 2014;28(2):83-89. doi:10.1097/BOT.0b013e31829e6dd0
17.
Little CP, Burston BJ, Hopkinson-Woolley J, Burge P. Failure of surgery for scaphoid non-union is associated with smoking. J Hand Surg Br. 2006;31(3):252-255. doi:10.1016/j.jhsb.2005.12.010
18.
Cobb TK, Gabrielsen TA, Campbell DC 2nd, Wallrichs SL, Ilstrup DM. Cigarette smoking and nonunion after ankle arthrodesis. Foot Ankle Int. 1994;15(2):64-67. doi:10.1177/107110079401500202
19.
van Houten AH, Heesterbeek PJ, van Heerwaarden RJ, van Tienen TG, Wymenga AB. Medial open wedge high tibial osteotomy: can delayed or nonunion be predicted? Clin Orthop Relat Res. 2014;472(4):1217-1223. doi:10.1007/s11999-013-3383-y
20.
Chen F, Osterman AL, Mahony K. Smoking and bony union after ulna-shortening osteotomy. Am J Orthop (Belle Mead NJ). 2001;30(6):486-489. PMID:11411875.
21.
Singh JA, Houston TK, Ponce BA, et al. Smoking as a risk factor for short-term outcomes following primary total hip and total knee replacement in veterans. Arthritis Care Res (Hoboken). 2011;63(10):1365-1374. doi:10.1002/acr.20555
22.
Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181. doi:10.1302/0301-620x85b2.13717
23.
Sandén B, Försth P, Michaëlsson K. Smokers show less improvement than nonsmokers two years after surgery for lumbar spinal stenosis: a study of 4555 patients from the Swedish spine register. Spine (Phila Pa 1976). 2011;36(13):1059-1064. doi:10.1097/BRS.0b013e3181e92b36
24.
Pearson A, Lurie J, Tosteson T, Zhao W, Abdu W, Weinstein JN. Who should have surgery for spinal stenosis? Treatment effect predictors in SPORT. Spine (Phila Pa 1976). 2012;37(21):1791-1802. doi:10.1097/BRS.0b013e3182634b04
25.
Myles PS, Iacono GA, Hunt JO, et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology. 2002;97(4):842-847. doi:10.1097/00000542-200210000-00015
26.
Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342. doi:10.2106/JBJS.I.00627
27.
Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117. doi:10.1016/S0140-6736(02)07369-5
28.
Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976). 2000;25(20):2608-2615. doi:10.1097/00007632-200010150-00011
29.
Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg. 2008;248(5):739-745. doi:10.1097/SLA.0b013e3181889d0d
30.
Hejblum G, Atsou K, Dautzenberg B, Chouaid C. Cost-benefit analysis of a simulated institution-based preoperative smoking cessation intervention in patients undergoing total hip and knee arthroplasties in France. Chest. 2009;135(2):477-483. doi:10.1378/chest.08-0897
31.
Boylan MR, Bosco JA 3rd, Slover JD. Cost-effectiveness of preoperative smoking cessation Interventions in total joint arthroplasty. J Arthroplasty. 2019;34(2):215-220. doi:10.1016/j.arth.2018.09.084
32.
Kapadia BH, Banerjee S, Cherian JJ, Bozic KL, Mont MA. The economic impact of periprosthetic infections after total hip arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2016;31(7):1422-1426. doi:10.1016/j.arth.2016.01.021
33.
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); 2014. PMID:24455788.
34.
Tosi LL, Gliklich R, Kannan K, Koval KJ. The American Orthopaedic Association's "own the bone" initiative to prevent secondary fractures. J Bone Joint Surg Am. 2008;90(1):163-173. doi:10.2106/JBJS.G.00682
35.
Bender D, Haubruck P, Boxriker S, Korff S, Schmidmaier G, Moghaddam A. Validity of subjective smoking status in orthopedic patients. Ther Clin Risk Manag. 2015;11:1297-1303. doi:10.2147/TCRM.S86212
36.
Helldán A, Helakorpi S. Health behaviour and health among the Finnish adult population, spring 2014. National institute for health and welfare (THL); 2015. Report 6/2015. ISBN 978-952-302-447-2. 2015. Accessed Augist 3, 2022.
https://www.julkari.fi/bitstre...
37.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127. doi:10.1111/j.1360-0443.1991.tb01879.x
38.
Kinnunen T, Korhonen T, Kaprio J. Mitä uutta tupakkariippuvuudesta ja sen hoidosta? Suomen Lääkärilehti [What is new about tobacco dependence and its treatment?]. Article in Finnish. Nordic Studies on Alcohol and Drugs. 2012;67(48):3559-3563. doi:10.2478/nsad-2014-0043
39.
Ehnert S, Aspera-Werz R, Ihle C, et al. Smoking dependent alterations in bone formation and inflammation represent major risk factors for complications following total joint arthroplasty. J Clin Med. 2019;8(3):406. doi:10.3390/jcm8030406
CITATIONS (7):
1.
Association of Smokeless Tobacco and Complications Following Ankle and Hindfoot Arthrodesis
Julianna E. Winter, Jacob S. Budin, Bela P. Delvadia, Matthew W. Cole, Timothy L. Waters, Adam P. Schiff, William F. Sherman
Foot & Ankle International
2.
Mitigating infections in implantable urological continence devices: risks, challenges, solutions, and future innovations. A comprehensive literature review
Bob Yang, Axelle Lavigne, Dario Carugo, Ben Turney, Bhaskar Somani, Eleanor Stride
Current Opinion in Urology
3.
Challenges in Orthopedic Surgical Decision-Making for Multilevel Vertebrae Fractures
Michelle O Uwefoh, Elizabeth Edah, Lee-Ann D Charles, Elvis Gini, Ekama Isong, Deborah A Ademo, Omavuaye J Eruvwedede, Daniel Ivankovich
Cureus
4.
A Scoring System for Predicting Nonunion After Intramedullary Nailing of Femoral Shaft Fractures
Kent R. Kraus, Joshua W. Flores, James E. Slaven, Ishani Sharma, Payton K. Arnold, Brian H. Mullis, Roman M. Natoli
JAAOS: Global Research and Reviews
5.
Epidemiology of complications after non-compulsory planned hardware-removal after limbs fracture
Guillaume Villatte, Arthur Haverlan, Marie Le Baron, Aurélien Mulliez, Stéphane Boisgard, Stéphane Descamps, Roger Erivan
Orthopaedics & Traumatology: Surgery & Research
6.
Épidémiologie des complications après ablation de matériel d’ostéosynthèse pour fracture des membres, programmée non obligatoire
Guillaume Villatte, Arthur Haverlan, Marie Le Baron, Aurélien Mulliez, Stéphane Boisgard, Stéphane Descamps, Roger Erivan
Revue de Chirurgie Orthopédique et Traumatologique
7.
Primary prevention in hospitals in 20 high-income countries in Europe – a case of not “making every contact count”?
Bernd Rechel, Béatrice Durvy, Gonçalo Figueiredo Augusto, Isabelle Aujoulat, Daiga Behmane, Anne-Carole Bensadon, Sara Burke, Melissa D'Agostino, Krisztina Davidovics, Mark Dayan, Antonio Giulio De Belvis, Judith de Jong, Katarzyna Dubas-Jakóbczyk, Inês Fronteira, Elena Gabriel, Giuseppe Greco, Peter Groenewegen, Signe Smith Jervelund, Marios Kantaris, Madelon Kroneman, Jerneja Farkas-Lainscak, Benjamin Maurice, Luisne Mac Conghail, Liubove Murauskiene, Mircha Poldrugovac, Zsuzsa Rákosy, Silvia Gabriela Scintee, Christoph Sowada, Frédéric Turblin, Desislava Vankova, Zita Velkey, Cristian Vladescu, Dorja Vocanec, Karsten Vrangbæk, Johannes Wünscher, Tuija Ylitörmänen
Health Policy



